Activated carbon is itself a typical porous carbon material. It features a highly developed pore structure, a large specific surface area, and excellent adsorption performance. It is widely used in adsorption, catalyst supports, and energy storage. Porous carbon is a broader concept that includes carbon materials with micro-, meso-, and macropores. In particular, hierarchical porous carbon exhibits a more complex pore architecture and optimized performance. Strictly speaking, activated carbon already belongs to the category of porous carbon. However, in both research and practical applications, commercial activated carbon or preliminarily carbonized materials are often used as precursors to prepare porous carbon with advanced structures. These materials are further treated through secondary activation or modification to produce hierarchical porous carbon with a higher specific surface area and a more optimized pore size distribution. This approach enables the development of micro-, meso-, and macroporous structures. As a result, mass transfer efficiency and overall performance are significantly improved in applications such as supercapacitors, electrocatalysis, and adsorption.
This article introduces the main methods, mechanisms, processing steps, and application prospects to prepare porous carbon based on activated carbon.

Differences Between Porous Carbon and Activated Carbon
- Activated carbon: Typically prepared by physical or chemical activation, dominated by micropores. The specific surface area usually ranges from 500 to 3000 m²/g. While highly porous, its pore size distribution is relatively simple.
- Porous carbon: A general term for carbon materials with various pore structures, particularly hierarchical porous carbon, which contains micropores (<2 nm, providing high surface area), mesopores (2–50 nm, facilitating mass transfer), and macropores (>50 nm, serving as transport channels). Such materials often exhibit higher surface areas and more optimized pore networks.
Using activated carbon as a precursor to prepare porous carbon is essentially a process of secondary activation or re-activation, aiming to further etch and tailor the pore structure.
Precursor Pretreatment: Ultrafine Grinding
Before secondary activation of activated carbon, ultrafine grinding is an important pretreatment step that can significantly improve activation efficiency and the performance of the resulting porous carbon.
Principle:
Commercial activated carbon is usually granular, with particle sizes ranging from tens to hundreds of micrometers. Although its internal pore structure is well developed, the diffusion of activating agents (such as KOH) is limited. Ultrafine grinding reduces particle size to the micron or even submicron scale (<10 μm), increasing the external surface area, exposing more active sites, and facilitating uniform impregnation and reaction with the activating agent. In addition, mechanical forces introduce defects into the carbon framework, enhancing its reactivity.
Common equipment:
- Ball mills: Planetary or vibratory ball mills, commonly used at laboratory and industrial scales.
- Jet mills or air classifier mills: Used for ultrafine grinding to obtain micron- or even nano-sized particles.
Effects and advantages:
- Finer particles lead to more uniform KOH impregnation; after activation, the specific surface area can increase by 20–50%, with a higher proportion of mesopores.
- Studies have shown that ball-milling pretreatment can optimize hierarchical pore structures and improve ion transport efficiency.

Preparation Methods
The main methods for preparing porous carbon from activated carbon precursors include chemical re-activation, template-assisted methods, and combined physical–chemical activation. Among them, KOH chemical re-activation is the most widely used.
KOH Chemical Re-Activation (Most Common)
Principle:
At high temperatures, KOH reacts with carbon to generate gases (such as CO and CO₂) and potassium-containing compounds, which etch the carbon framework and create new pores. Simultaneously, potassium vapor intercalates between carbon layers, further expanding the pore structure.
Simplified reaction mechanisms:
- 6KOH + 2C → 2K + 3H₂ + 2K₂CO₃
- K₂CO₃ → K₂O + CO₂
- Subsequent reduction reactions generate metallic K, further enlarging pores.
Process steps (combined with ultrafine grinding):
- Ultrafine grinding of activated carbon to obtain fine powder.
- Mixing the ultrafine activated carbon with a KOH solution (typical KOH/carbon mass ratio: 1:1 to 4:1) and stirring or milling thoroughly.
- Drying, followed by high-temperature activation under an inert atmosphere (N₂ or Ar) at 600–900 °C for 1–3 hours.
- Cooling, then washing with dilute acid (e.g., HCl) to remove residual potassium compounds, followed by rinsing with water until neutral.
- Drying to obtain hierarchical porous carbon.
Key influencing factors:
- KOH ratio: Higher ratios increase surface area, but excessive KOH may cause structural collapse.
- Activation temperature: Around 800 °C is often optimal; higher temperatures favor mesopore formation.
- Activation time: Excessively long times may over-etch carbon and reduce yield.
- Pre-grinding: Significantly improves activation uniformity.
Typical performance:
Hierarchical porous carbon with a specific surface area >2000 m²/g and pore volume >1 cm³/g can be obtained, widely used as supercapacitor electrodes.
Other Chemical Activators
ZnCl₂ or H₃PO₄: Suitable for further development of mesopores, though with lower yield.
K₂CO₃: A milder activator, suitable for preparing porous carbon with higher graphitization.
Template-Assisted Re-Activation
Activated carbon can be combined with hard templates (e.g., SiO₂ nanoparticles, MgO) or soft templates (surfactants), followed by KOH activation.
- Process: Impregnation of activated carbon with template and KOH → high-temperature carbonization → template removal (HF or acid washing).
- Advantages: More ordered pore structures and better control over meso- and macropore ratios.
Physical Re-Activation
Secondary activation using CO₂ or steam at high temperatures can further develop micropores, but the efficiency is generally lower than that of chemical methods.
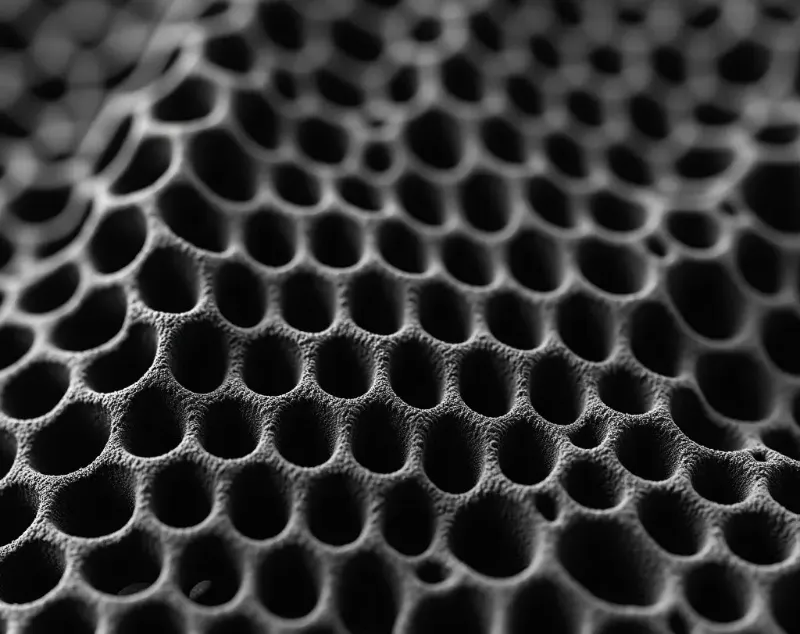
Typical Cases and Performance
- Coal-based activated carbon, after ultrafine grinding and KOH re-activation, can yield hierarchical porous carbon with surface areas up to 3000 m²/g, suitable for oxygen reduction reaction (ORR) electrocatalysis.
- Biomass-derived activated carbon (e.g., coconut shell carbon), after re-activation, can produce hierarchical porous carbon with specific capacitances of 300–400 F/g in supercapacitors.
- Studies show that re-activated materials often exhibit honeycomb-like hierarchical pore structures, which are beneficial for ion transport and gas diffusion.
Application Prospects
- Energy storage: Supercapacitors, lithium/sodium-ion battery anodes.
- Electrocatalysis: Oxygen evolution reaction (OER) and oxygen reduction reaction (ORR).
- Adsorption and separation: CO₂ capture, heavy metal removal, dye adsorption.
- Environmental sustainability: Re-activation of waste activated carbon for resource recycling.
Conclusion
Using activated carbon as a precursor to prepare porous carbon is an effective secondary processing approach, especially when combined with ultrafine grinding and KOH chemical re-activation. Ultrafine grinding plays a key role in improving activation uniformity and pore structure development.
Epic Powder’s ultrafine grinding equipment, including ball mills and air classifier mills, can reduce activated carbon to micron or submicron sizes, enhancing KOH diffusion and reaction efficiency. This enables the stable production of hierarchical porous carbon with high specific surface area and optimized pore size distribution.
With reliable and scalable powder processing solutions, Epic Powder supports the industrial preparation of high-performance porous carbon for energy storage, catalysis, and adsorption applications.

“Thanks for reading. I hope my article helps. Please leave a comment down below. You may also contact Zelda online customer representative for any further inquiries.”
— Posted by Emily Chen
