Titanato de bario (BaTiO₃) El polvo es la materia prima principal de la cerámica electrónica a base de titanato. Como material ferroeléctrico típico con excelentes propiedades dieléctricas, se utiliza ampliamente en condensadores cerámicos multicapa (MLCC), dispositivos de sonar, detectores de radiación infrarroja, condensadores cerámicos de límite de grano y termistores de coeficiente de temperatura positivo (PTC). Con amplias posibilidades de aplicación, el titanato de bario se considera un material fundamental de la cerámica electrónica.
Con la tendencia actual hacia la miniaturización, el diseño liviano, la alta confiabilidad y los componentes electrónicos delgados, la demanda de alta pureza y polvo ultrafino de titanato de bario se ha vuelto cada vez más urgente.
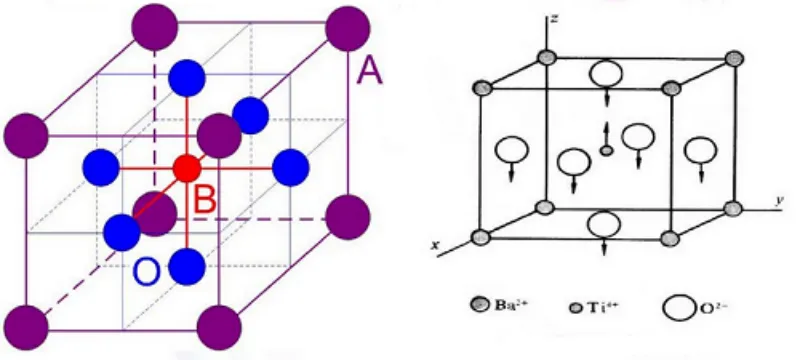
Descripción general del titanato de bario
El titanato de bario es un compuesto de fusión congruente con un punto de fusión de 1618 °C. Presenta cinco polimorfos cristalinos: hexagonal, cúbico, tetragonal, ortorrómbico y romboédrico. A temperatura ambiente, la fase tetragonal es termodinámicamente estable.
Ferroelectricidad del titanato de bario
Cuando el BaTiO₃ se somete a un campo eléctrico intenso, se produce una polarización persistente por debajo de su temperatura de Curie, de aproximadamente 120 °C. El titanato de bario polarizado presenta dos propiedades clave: ferroelectricidad y piezoelectricidad.
En los cristales ferroeléctricos de BaTiO₃, existen numerosas regiones pequeñas en las que difieren las direcciones de polarización espontánea. Cada región consta de numerosas celdas unitarias con la misma dirección de polarización; estas regiones se conocen como dominios. Los cristales con estas estructuras de dominio se denominan cristales ferroeléctricos o ferroeléctricos. Bajo un campo eléctrico externo, el tamaño y la geometría de estos dominios cambian consecuentemente.
Temperatura de Curie del titanato de bario
La temperatura de Curie (Tc) del BaTiO₃ se refiere a la temperatura de transición de fase entre las fases tetragonal y cúbica, a la cual el cristal ferroeléctrico pierde su polarización espontánea y desaparece la estructura del dominio. La temperatura de Curie del BaTiO₃ es de aproximadamente 120 °C.

Métodos de preparación de polvo de titanato de bario
Los métodos de preparación de polvo de titanato de bario generalmente se pueden dividir en tres categorías: método de estado sólido, método hidrotérmico y método sol-gel.
Método de estado sólido
El método de estado sólido, también conocido como síntesis en fase sólida a alta temperatura, es el método más clásico para la preparación de polvos de titanato de bario. Su principio básico consiste en reacciones controladas por difusión entre materias primas sólidas a temperaturas elevadas.
Normalmente, el carbonato de bario (BaCO₃) y el dióxido de titanio (TiO₂) se mezclan según proporciones estequiométricas, seguido de molienda y peletización o calcinación a alta temperatura (generalmente 1100-1300 °C) durante varias horas para inducir una reacción en estado sólido y formar polvo de BaTiO₃. La reacción es la siguiente:
BaCO₃ + TiO₂ → BaTiO₃ + CO₂ ↑
Este método, que se caracteriza por su equipo sencillo y su bajo costo, se ha adoptado ampliamente para la producción industrial a gran escala. Sin embargo, los polvos resultantes suelen tener tamaños de partícula relativamente grandes (escala micrométrica) y tienden a presentar aglomeración y contaminación por impurezas.
· Aplicación de equipos de molienda

- Molino de bolas: Se utiliza durante la etapa de dosificación para mezclar uniformemente las materias primas y reducir tamaño de partícula, aumentando así el área de contacto.
- Molino de cuentas: Después de la calcinación, el titanato de bario a menudo forma aglomerados duros; los molinos de perlas horizontales se utilizan comúnmente para una molienda intensiva para obtener productos micrométricos o submicrónicos.
· Ventajas y desventajas:
Bajo costo y alto rendimiento, pero propenso a introducir impurezas inducidas por desgaste y producir polvos relativamente gruesos.
Método hidrotermal
El método hidrotermal es una técnica de síntesis en fase líquida que se lleva a cabo en soluciones acuosas a alta temperatura y alta presión, y se utiliza ampliamente para preparar polvos de titanato de bario a escala nanométrica.
En este proceso, se disuelven sales de bario (como el hidróxido de bario) y de titanio (como el cloruro de titanio) en agua, con la adición de mineralizadores (p. ej., NaOH). La mezcla se somete a reacción en un autoclave hidrotermal a 150-250 °C a alta presión durante varias horas, obteniéndose directamente polvos de BaTiO₃ bien cristalizados.
Este método no requiere calcinación a alta temperatura y permite un control preciso del tamaño de partícula (normalmente de 50 a 200 nm), con alta cristalinidad y pureza de fase (tetragonal o cúbica). Además, es respetuoso con el medio ambiente. Sin embargo, requiere equipos sofisticados y un control estricto de las condiciones de reacción.
· Aplicación de equipos de molienda

- Dispersión de precursores: Antes del tratamiento en autoclave, a menudo se utilizan molinos de vibración o molinos de bolas para garantizar una dispersión homogénea de la suspensión.
- Desaglomeración post-tratamiento: Aunque los nanopolvos sintetizados hidrotermalmente tienen una alta cristalinidad, puede ocurrir una aglomeración suave durante el secado. Molinos de chorro se utilizan comúnmente en esta etapa. Mediante colisiones entre partículas sin medios de molienda, fresado por chorro Rompe eficazmente los aglomerados evitando la contaminación metálica y preservando las características a nanoescala.
· Ventajas y desventajas:
Pureza extremadamente alta y tamaño de partícula nanométrica, lo que lo convierte en el método preferido para la producción de MLCC de alta gama.
Método Sol-Gel
El método sol-gel es un tipo de síntesis en fase líquida que permite la preparación de polvos con control molecular. Se utilizan como precursores alcóxidos de titanio (como el titanato de tetrabutilo) y sales de bario (como el acetato de bario). Mediante hidrólisis en un disolvente orgánico, se forma un sol, que posteriormente se transforma en un gel por evaporación o calentamiento. Tras el secado y la calcinación a baja temperatura (600-900 °C), se obtiene el polvo de BaTiO₃.
Este método produce polvos con tamaño de partícula nanométrico, alta pureza y excelente uniformidad compositiva, lo que lo hace adecuado para cerámicas electrónicas de alto rendimiento. Sin embargo, las materias primas son caras y se requiere un control estricto del pH y la temperatura para evitar precipitaciones no homogéneas.
· Aplicación de equipos de molienda
- Planetario Molino de bolas: El gel seco obtenido mediante el proceso sol-gel es extremadamente frágil. Para obtener nanopolvos uniformes, se suele emplear la molienda seca o húmeda de corta duración con un molino de bolas planetario.
· Ventajas y desventajas:
Este método ofrece la mejor uniformidad de composición, pero debido a los altos costos de la materia prima, la toxicidad de los solventes, la rápida aglomeración durante el tratamiento térmico y los estrictos requisitos de control del proceso, es difícil de industrializar y actualmente se limita principalmente a la investigación de laboratorio y a aplicaciones especializadas de película delgada.
Conclusión
Los tres métodos principales de preparación de polvo de titanato de bario (estado sólido, sol-gel e hidrotérmico) presentan ventajas y limitaciones específicas. El método de estado sólido es adecuado para la producción a gran escala, pero produce polvos relativamente gruesos. Por el contrario, los métodos sol-gel e hidrotérmicos pueden producir polvos a escala nanométrica y son más adecuados para aplicaciones electrónicas de alta gama.
El equipo de molienda desempeña un papel indispensable en todos estos métodos: es esencial para la mezcla de materias primas y el refinamiento de partículas en la síntesis en estado sólido, y facilita la dispersión posterior al tratamiento en procesos en fase líquida. Al optimizar los parámetros de molienda, como los materiales del medio de molienda, la velocidad de rotación y el tiempo de molienda, se puede mejorar significativamente la calidad y el rendimiento de los polvos de titanato de bario.
De cara al futuro, con los avances en las tecnologías de molienda y dispersión, en particular la introducción de equipos de molienda a escala nanométrica, la preparación de polvos de titanato de bario será más eficiente, lo que impulsará aún más la innovación en la industria de los materiales electrónicos.

Gracias por leer. Espero que mi artículo te haya sido útil. Deja un comentario a continuación. También puedes contactar con el servicio de atención al cliente online de Zelda para cualquier otra consulta.
— Publicado por Emily Chen
