Morphology control of powder particles is one of the core technologies in advanced material preparation. It directly determines the packing density, flowability, sintering activity, and the final microstructure and performance of ceramic products. The objective of morphology control is to obtain particle shapes that are specific, uniform, and reproducible.
The following sections detail the mainstream preparation methods and the underlying principles behind these approaches to achieve powder particles control.
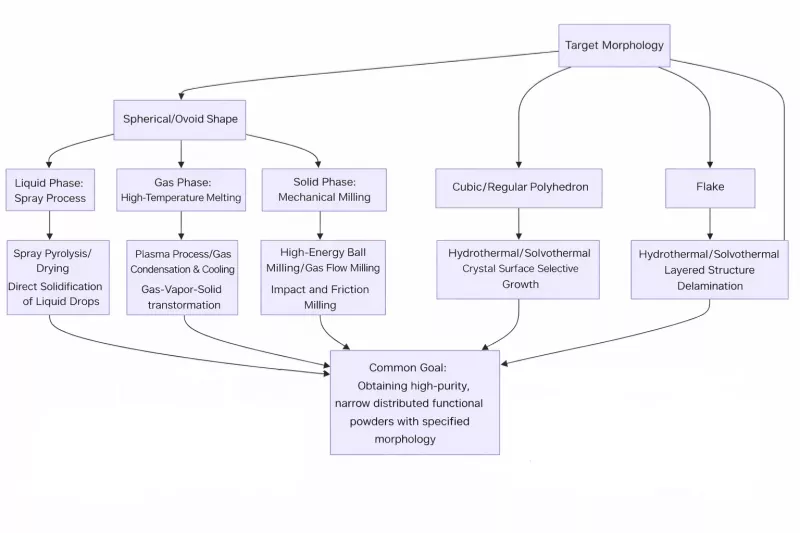
Mainstream Morphologies and Control Strategies
The following table details the characteristics, common preparation methods, and core control principles of different target morphologies.
Morphology Control Strategy Table
| Target Morphology | Characteristics & Advantages | Typical Preparation Methods | Core Principles of Control |
| Spherical / Near-Spherical | High packing density, excellent fluidity, low sintering activity. Facilitates high solid content, low viscosity, and uniform green bodies in slurry preparation (e.g., tape casting). | 1. Spray Methods: Spray pyrolysis, spray drying. 2. Gas Phase: RF plasma, hóa chất vapor condensation. 3. Liquid Phase: Homogeneous precipitation + calcination. 4. Mechanical: High-energy máy nghiền bi (rounding). | 1. Surface Tension Dominance: Droplets or molten matter naturally contract into spheres under surface tension. 2. Interfacial Energy Minimization: Controlling reaction rates for isotropic growth. 3. Mechanical Shaping: Rounding sharp edges through collision and friction. |
| Cubic / Regular Polyhedron | Complete crystal structure, controllable anisotropy. For perovskites like BaTiO3, cubic particles pack tightly, reducing sintering stress and improving MLCC reliability. | Hydrothermal / Solvothermal methods are the most classic and effective routes. | Crystallographic Anisotropic Growth: Precisely controlling temperature, pressure, time, mineralizers (e.g., OH-), and surfactants to inhibit or promote specific crystal planes (e.g., {100}), allowing particles to develop along thermodynamically stable directions. |
| Plate-like / Layered | Distinct 2D structure. Used for textured ceramics (e.g., piezoelectrics) to boost directional performance; also used as barriers in coatings or composites. | 1. Hydrothermal/Solvothermal (using layered precursors). 2. Molten Salt Method. 3. Exfoliation (e.g., exfoliating Layered Double Hydroxides – LDH). | 1. Intrinsic Structural Guidance: Ensuring growth occurs primarily within the 2D plane while inhibiting thickness growth. 2. Template Direction: Epitaxial growth on plate-like templates (e.g., mica). 3. Molten Salt Media: Providing a 2D constrained space. |
| Core-Shell / Hollow Structure | Multi-functional composites, high specific surface area, lightweight. Used in catalysts, drug delivery, and high-performance electrode materials. | 1. Templating (Hard/Soft templates). 2. Ostwald Ripening. 3. Layer-by-Layer (LbL) Self-assembly. | 1. Template Confinement: Lớp phủ target materials onto a spherical template, then removing the template. 2. Diffusion Control: Utilizing the differential diffusion rates of internal and external substances to form cavities (Kirkendall effect). |

Universal Elements of Morphology Control
Regardless of the method, effective powder particles control relies on the precise regulation of several key factors::
- Thermodynamic vs. Kinetic Balance:
- Thermodynamic Control: Under conditions near equilibrium (e.g., long-duration, low-temp hydrothermal), particles tend toward regular, low-surface-energy shapes (e.g., cubes).
- Kinetic Control: Under far-from-equilibrium conditions (e.g., rapid precipitation, high-temp spray), particles form non-equilibrium shapes (e.g., spheres, dendrites). Adjusting reaction rates (concentration, temperature) allows switching between these regimes.
- Surface Energy & Crystal Plane Specificity:Different crystal planes have varying surface energies. Additives (surfactants, chelating agents) can selectively adsorb onto specific high-energy planes, inhibiting their growth and exposing desired facets. Example: PVP is often used to induce the growth of silver nanorods.
- Separation of Nucleation and Growth:“Burst nucleation” is a vital strategy. By instantaneously creating extreme supersaturation, a massive number of nuclei form simultaneously. Subsequent controlled growth ensures these nuclei develop uniformly, resulting in monodisperse particles with consistent morphology.
- Reaction Environment and Media:
- Solvent: Polarity affects reactant solubility and diffusion rates.
- pH Value: Influences the chemical form and reactivity of precursors.
- Mineralizers: In hydrothermal synthesis, strong bases (like $NaOH$) act as mineralizers, increasing precursor solubility and altering the relative growth rates of different crystal planes.
Summary and Industry Significance
Powder morphology control serves as the bridge connecting molecular/atomic synthesis chemistry to macroscopic material performance.
- For Electronic Ceramics: Cubic barium titanate (BaTiO3) is the standard for high-end MLCCs; spherical alumina/aluminum nitride is the foundation for high-performance thermal fillers.
- For Catalysis & Energy: High-surface-area porous or hollow structures expose more active sites.
- For Biomedicine: Specific particle shapes influence circulation time and targeting efficiency within the body.
Mastering morphology control means the ability to “customize” the primary structure of materials—an essential path toward high-performance and functionalized materials. Future trends point toward greener, more precise, and scalable techniques (such as continuous flow reactors) and a deeper understanding of the “morphology-performance” correlation.

Cảm ơn bạn đã đọc. Tôi hy vọng bài viết của tôi hữu ích. Vui lòng để lại bình luận bên dưới. Bạn cũng có thể liên hệ với bộ phận chăm sóc khách hàng trực tuyến của Zelda nếu có bất kỳ thắc mắc nào khác.
— Đăng bởi Emily Chen
